Wearable electronics have been gaining traction in the medical device industry in recent years, and for good reason. They are perfect for a variety of applications, and benefits ranging from improving patient care and outcomes to streamlining manufacturing processes. Let’s look at how wearable electronics are revolutionizing healthcare and some of the design and manufacturing considerations that come along with them.
What are Wearable Electronics?
Wearable electronics are small, battery-powered electronic devices that can be worn on the body and used to monitor activities and vital signs. These devices are typically lightweight and have a slim profile, allowing them to be worn comfortably and discreetly, such as patient-worn patches. You may have seen wearable electronics used to track heart rate, activity levels, blood pressure, and other vital signs. They can also be used to record sleep patterns, alert users to changes in their environment, and even monitor medication adherence.
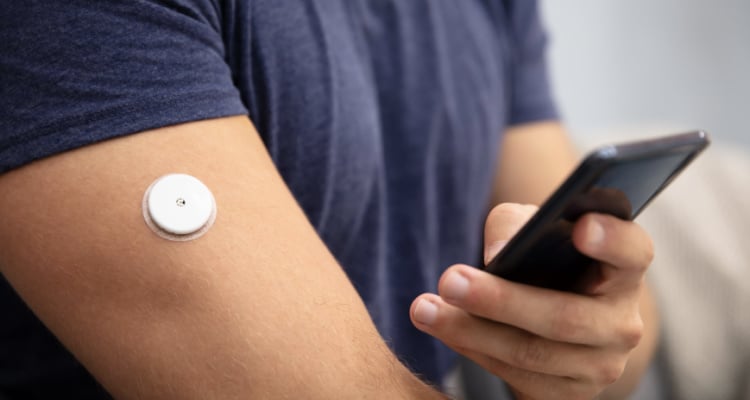
Benefits of Wearable Electronics in the Medical Device Industry
The use of wearable electronics in the medical device industry offers many advantages. For example, clinicians can view more detailed and accurate information about a patient’s health and wellbeing. This is particularly helpful for those with chronic conditions who require regular doses of medication, sending an alert when a dose is missed. As a major benefit for the provider, wearable electronics can be used to collect data that can track the effectiveness of treatments, identify potential health issues, and provide valuable insights into patient behavior and improve patient outcomes. Additionally, they can monitor the safety and performance of medical devices in real-time, reducing the risk of product defects and malfunctions. However, the development of a successful device first depends on creating a great design.
Wearable Electronics Design Considerations
Sure, the design needs to meet desired specifications, requirements, and regulations, but the best engineers will map out the product’s entire lifespan from production to final disposal. This includes early and proper execution of DFM (design for manufacturing) methods to simplify, optimize and refine the design, resulting in a better product at a lower cost. Best practices suggest utilizing a cross-functional DFM team, including engineers, designers, manufacturers, and material suppliers, in order to challenge the design and look at it through every lens and from every level of the manufacturing process.
We know it is important to make sure the device is both comfortable and durable, but engineers must first ensure they are selecting the right components and maHow terials so the device is both safe and effective. Even decisions that may seem unimportant on the surface, such as which adhesive to use, must be made intentionally as it can influence overall performance. What environmental conditions will this product be encountering? How rugged should it be? What about its flexibility?
It’s common to see wearable electronics that must be designed to have a long battery life. This is especially important for medical applications, as the device must be able to run for an extended period of time without needing to be recharged. Oh, and don’t forget the device must be designed to be easy to use, with a user interface that is intuitive and user-friendly.
This data can be used to track the effectiveness of treatments, identify potential health issues, and provide valuable insights into patient behavior. Additionally, wearable electronics can be used to provide real-time feedback to patients, allowing them to better manage their health and wellbeing.
Wearable Electronics and Medical Device Regulation
It’s worth quickly mentioning that the use of wearable electronics in the medical device industry is subject to regulation by the FDA. The FDA has established strict guidelines and regulations for the design, manufacture, and marketing of medical devices, so it is important for manufacturers to ensure their devices meet these requirements to guarantee the safety and effectiveness of the device. While looking for a supplier, first ensure their facility is ISO 13485 certified.
From improved patient care and outcomes to streamlined manufacturing processes, wearable electronics are providing numerous advantages for the medical device industry. However, there are also a number of challenges and considerations that must be taken into account when designing and manufacturing wearable electronics. By implementing early DFM practices and following the appropriate regulatory requirements, manufacturers can ensure their devices are safe, effective, and compliant.




.jpg?width=176&height=56&name=MR_associatedNetwork_logo%20(1).jpg)